
It can be purified, but the process is expensive. Glycerol from triglycerides is produced on a large scale, but the crude product is of variable quality, with a low selling price of as low as US$0.02–0.05 per kilogram in 2011. It was projected in 2006 that by 2020, production would be six times more than demand, creating an excess of glycerol as a byproduct of biofuel production. The EU directive 2003/30/EC set a requirement that 5.75% of petroleum fuels were to be replaced with biofuel sources across all member states by 2010. Approximately 950,000 tons per year are produced in the United States and Europe 350,000 tons of glycerol were produced per year in the U.S. Typical plant sources include soybeans or palm. Triglycerides can be saponified with sodium hydroxide to give glycerol and fatty sodium salt or soap.
The hydrolysis, saponification, or transesterification of these triglycerides produces glycerol as well as the fatty acid derivative: Glycerol is generally obtained from plant and animal sources where it occurs in triglycerides, esters of glycerol with long-chain carboxylic acids. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a sn- prefix before the stem name of the molecule. Structure Īlthough achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Its presence in blood can be used as an effective marker to measure liver disease. Conversely, it is also used as a bacterial culture medium. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. The glycerol backbone is found in lipids known as glycerides.
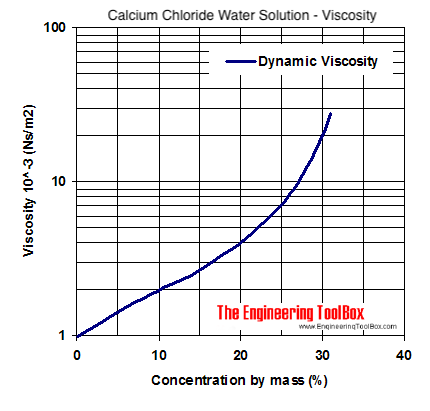
It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. These factors are of particular relevance to an understanding of the response of cells such as spermatozoa, red blood cells, and bacteria cooled rapidly with glycerol as cryoprotectant.Glycerol ( / ˈ ɡ l ɪ s ə r ɒ l/), also called glycerine or glycerin, is a simple triol compound. At a critical rate of cooling, water diffusion becomes limited by the high viscosity and two phenomena, of relevance to cryobiology, occur: (1) the composition of the freeze concentrated matrix around cells deviates from that of the equilibrium phase diagram and (2) the osmotic loss of water from cells is restricted. Validation of the diffusion calculations was confirmed by examination of the ultrastructure of the freeze concentrated matrix in samples prepared at a range of cooling rates. At rates of cooling faster than 100 degrees C min(-1) the diffusion distance during freezing was calculated to be less than 15 microm. The effect of these high viscosities on the diffusion of water at a constant temperature during freezing and during cooling at different linear rates has been estimated. The viscosity of the glycerol-water binary system exceeded 1000 cP at -40 degrees C, whilst the viscosity of the ternary system, glycerol-water-NaCl, exceeded 100,000 cP at -55 degrees C.

The objective of this study was to determine the viscosity of the residual unfrozen solution that cells are exposed to during freezing in the presence of glycerol and use this to interpret some key aspects of cryopreservation.


 0 kommentar(er)
0 kommentar(er)
